Abstract
T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL/TLBL) is an aggressive malignancy of immature T-Cells which primarily affects children and young adults. Although B-ALL treatments have improved dramatically, success has been more limited in T-ALL. First line treatment consists of multi-agent chemotherapy with 80-90% of patients achieving a complete remission but 30-40% patients will relapse. The prognosis of relapsed T-ALL is poor with survival rates of less than 25%. Allogeneic stem cell transplant is the only curative modality. The incidence and outcomes of T-ALL have not been well studied in predominantly minority populations.
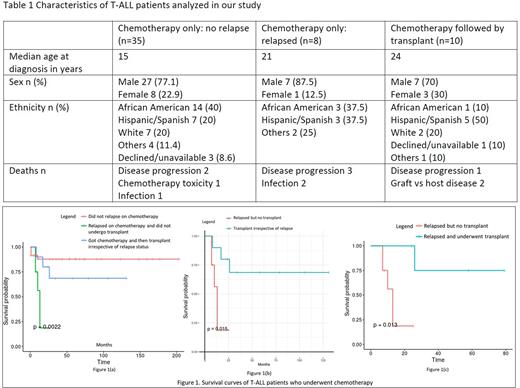
Here we report a single-center retrospective observational study investigating patients diagnosed with T-ALL at the Montefiore Medical Center from 2004 until 2022. We identified 59 unique records with this diagnosis. Seventy-eight percent were males and 22% were females. The median age at diagnosis was 20 years (range 1-48 years). Thirty two percent of patients were African American, 29% were Hispanic, and 17% were white (Table 1). Patients were treated at diagnosis with conventional chemotherapy regimens including Hyper CVAD (n=12), CALGB 10403 (n=9), COG AALL00P2 (n=2),COG AALL0434 (n=5), COG AALL1231 (n=7), E2993 (n=1),CCG 1961 (n=2), DFCI 11-001 (n=9), DFCI 05-001 (n=4), DFCI 16-001 (n=3), POG 69404 MSKCC NY II protocol (n=1) and unknown (n=4). Fifty-three patients had available data following chemotherapy and were selected for analysis. Thirty-five patients (66%) achieved a complete remission with first-line therapy and did not relapse. Five patients (9.5%) underwent transplant in first remission and did not relapse. Eight (15.1%) of the thirteen patients (24.5%) who relapsed received further lines of chemotherapy but no transplant while the remaining five (9.5%) received a transplant.
Our transplant cohort was composed of 10 patients, eight of which received an allogeneic graft (two matched related donor transplant, four received matched unrelated, one haploidentical, and one cord blood transplant) and two an autologous graft. Two of the transplanted patients received a second transplant (one haploidentical and one matched related donor). Median overall survival was not reached in the cohort of patients that were responsive to chemotherapy or in those who received a transplant. On the contrary, median overall survival of the patients who relapsed on chemotherapy was 13 months.
The overall mortality rate of our cohort was 20.3% (12/59). Eighty-nine percent (32/35) of those who did not relapse after receiving chemotherapy survived. Seventy percent (7/10) of patients who received chemotherapy and a transplant survived while only 37.5% (3/8) of patients who relapsed on chemotherapy and did not get a transplant survived. The cause of death of the whole cohort including transplants was progression of disease in 6/12 (50%), infection in 3/12 (25%), graft vs host disease in 2/12 (16.7%), and toxicity of chemotherapy in 1/12 (8.3%). Among the three patients who died after receiving transplant, two of them died due to graft vs host disease while the third patient relapsed after transplant and died because of disease progression.
This is the first report of long-term outcomes of T-ALL in a mainly minority urban population in NYC. Our results indicate that most patients can be cured with chemotherapy alone (figure 1(a)). Nevertheless, chemotherapy refractoriness or relapse portends a dismal outcome. Remarkably, patients who relapsed but underwent stem cell transplantation performed significantly better (p=0.013) than patients who relapsed but did not undergo transplant (figure 1(c)). Similarly, the patient cohort receiving stem cell transplant irrespective of relapse after chemotherapy performed significantly better (p=0.015) than those who did not receive transplant after relapsing on chemotherapy (figure 1(b)). Hence, stem cell transplant should be considered to patients upon relapse based on our findings. Studies on larger cohorts of patients are needed to provide a better understanding on the role of allogeneic transplantation in relapsed T-ALL.
Disclosures
Bazarbachi:ASH: Research Funding. Shastri:Janssen: Consultancy; Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; NACE: Honoraria; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Verma:Incyte: Research Funding; MedPacto: Research Funding; Celgene: Consultancy, Research Funding; GlaxoSmithKline: Research Funding; Curis: Research Funding; Stelexis Therapeutics: Current equity holder in publicly-traded company; BMS: Research Funding; Jannsen: Research Funding; Novartis: Consultancy, Research Funding; Acceleron Pharma: Consultancy; Stelexis Therapeutics: Consultancy; Eli Lilly and Company: Research Funding; Throws Exception: Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal